

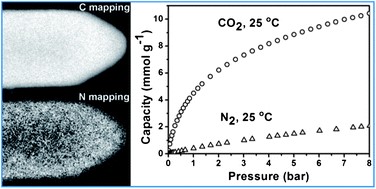
 Nitrogen-doped carbon materials were prepared by a nanocasting route using tri-continuous mesoporous silica IBN-9 as a hard template. Rationally choosing carbon precursors and carefully controlling activation conditions result in an optimized material denoted as IBN9-NC1-A, which possesses a very high nitrogen doping concentration (∼13 wt%) and a large surface area of 890 m2 g−1 arising from micropores (<1 nm). It exhibits an excellent performance for CO2 adsorption over a wide range of CO2 pressures. Specifically, its equilibrium CO2 adsorption capacity at 25 °C reaches up to 4.50 mmol g−1 at 1 bar and 10.53 mmol g−1 at 8 bar. In particular, it shows a much higher CO2 uptake at low pressure (e.g. 1.75 mmol g−1 at 25 °C and 0.2 bar) than any reported carbon-based materials, owing to its unprecedented nitrogen doping level. The high nitrogen contents also give rise to significantly enhanced CO2/N2 selectivities (up to 42), which combined with the high adsorption capacities, make these new carbon materials promising sorbents for selective CO2 capture from power plant flue gas and other relevant applications.
Nitrogen-doped carbon materials were prepared by a nanocasting route using tri-continuous mesoporous silica IBN-9 as a hard template. Rationally choosing carbon precursors and carefully controlling activation conditions result in an optimized material denoted as IBN9-NC1-A, which possesses a very high nitrogen doping concentration (∼13 wt%) and a large surface area of 890 m2 g−1 arising from micropores (<1 nm). It exhibits an excellent performance for CO2 adsorption over a wide range of CO2 pressures. Specifically, its equilibrium CO2 adsorption capacity at 25 °C reaches up to 4.50 mmol g−1 at 1 bar and 10.53 mmol g−1 at 8 bar. In particular, it shows a much higher CO2 uptake at low pressure (e.g. 1.75 mmol g−1 at 25 °C and 0.2 bar) than any reported carbon-based materials, owing to its unprecedented nitrogen doping level. The high nitrogen contents also give rise to significantly enhanced CO2/N2 selectivities (up to 42), which combined with the high adsorption capacities, make these new carbon materials promising sorbents for selective CO2 capture from power plant flue gas and other relevant applications.