

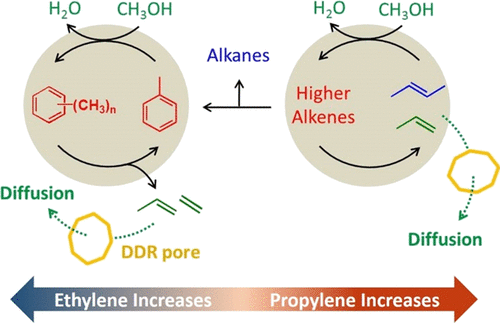
 Zeolites that have ultrasmall pore sizes usually exhibit high selectivity toward light olefins when used as catalysts for the conversion of methanol to hydrocarbons (MTH). However, tuning the selectivity of a desired product in a controllable manner remains difficult. In this study, we performed MTH reactions on DDR zeolites at 400 °C and subcomplete methanol conversions to investigate how the highly constrained pore structure of a DDR zeolite impacts on the reaction pathways and the product selectivity. We find that the propylene selectivity monotonically increases as the aluminum content and crystallize size of the DDR zeolite decreases. With this understanding, we are able to maximize the propylene selectivity up to 50.6% by modifying the DDR zeolite. We performed isotopic tracing experiments and analysis of residual species in the catalysts to validate the dual-cycle (aromatic- and olefin-based cycle) mechanism in DDR zeolite-catalyzed MTH and demonstrate that propylene is primarily produced from the olefin-based cycle. We attribute the unique behavior of DDR zeolites to that their ultrasmall pore sizes impose restriction on the molecular diffusion of higher olefins, leading to a more important role of the olefin-based catalytic cycle in the reaction, in comparison with other zeolites with larger pores.
Zeolites that have ultrasmall pore sizes usually exhibit high selectivity toward light olefins when used as catalysts for the conversion of methanol to hydrocarbons (MTH). However, tuning the selectivity of a desired product in a controllable manner remains difficult. In this study, we performed MTH reactions on DDR zeolites at 400 °C and subcomplete methanol conversions to investigate how the highly constrained pore structure of a DDR zeolite impacts on the reaction pathways and the product selectivity. We find that the propylene selectivity monotonically increases as the aluminum content and crystallize size of the DDR zeolite decreases. With this understanding, we are able to maximize the propylene selectivity up to 50.6% by modifying the DDR zeolite. We performed isotopic tracing experiments and analysis of residual species in the catalysts to validate the dual-cycle (aromatic- and olefin-based cycle) mechanism in DDR zeolite-catalyzed MTH and demonstrate that propylene is primarily produced from the olefin-based cycle. We attribute the unique behavior of DDR zeolites to that their ultrasmall pore sizes impose restriction on the molecular diffusion of higher olefins, leading to a more important role of the olefin-based catalytic cycle in the reaction, in comparison with other zeolites with larger pores.