

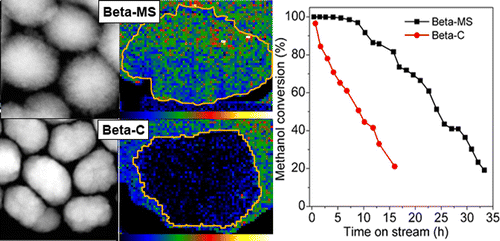
 Hierarchically porous zeolite Beta (Beta-MS) synthesized by a soft-templating method contains remarkable intracrystalline mesoporosity, which reduces the diffusion length in zeolite channels down to several nanometers and alters the distribution of Al among distinct crystallographic sites. When it was used as a catalyst for the conversion of methanol to hydrocarbons (MTH) at 330 °C, Beta-MS exhibited a 2.7-fold larger conversion capacity, a 2.0-fold faster reaction rate, and a remarkably longer lifetime in comparison to conventional zeolite beta (Beta-C). The superior catalytic performance of Beta-MS is attributed to its hierarchical structure, which offers full accessibility to all catalytically active sites. In contrast, Beta-C was easily deactivated because a layer of coke quickly deposited on the outer surfaces of the catalyst crystals, impeding access to interior active sites. This difference is clearly demonstrated by using electron microscopy combined with electron energy loss spectroscopy to probe the distribution of coke in the deactivated catalysts. At both low and high conversions, ranging from 20% to 100%, Beta-MS gave higher selectivity toward higher aliphatics (C4–C7) but lower ethene selectivity in comparison to Beta-C. Therefore, we conclude that a hierarchical structure decreases the residence time of methylbenzenes in zeolite micropores, disfavoring the propagation of the aromatic-based catalytic cycle. This conclusion is consistent with a recent report on ZSM-5 and is also strongly supported by our analysis of soluble coke species residing in the catalysts. Moreover, we identified an oxygen-containing compound, 4-methylbenzaldehyde, in the coke, which has not been observed in the MTH reaction before.
Hierarchically porous zeolite Beta (Beta-MS) synthesized by a soft-templating method contains remarkable intracrystalline mesoporosity, which reduces the diffusion length in zeolite channels down to several nanometers and alters the distribution of Al among distinct crystallographic sites. When it was used as a catalyst for the conversion of methanol to hydrocarbons (MTH) at 330 °C, Beta-MS exhibited a 2.7-fold larger conversion capacity, a 2.0-fold faster reaction rate, and a remarkably longer lifetime in comparison to conventional zeolite beta (Beta-C). The superior catalytic performance of Beta-MS is attributed to its hierarchical structure, which offers full accessibility to all catalytically active sites. In contrast, Beta-C was easily deactivated because a layer of coke quickly deposited on the outer surfaces of the catalyst crystals, impeding access to interior active sites. This difference is clearly demonstrated by using electron microscopy combined with electron energy loss spectroscopy to probe the distribution of coke in the deactivated catalysts. At both low and high conversions, ranging from 20% to 100%, Beta-MS gave higher selectivity toward higher aliphatics (C4–C7) but lower ethene selectivity in comparison to Beta-C. Therefore, we conclude that a hierarchical structure decreases the residence time of methylbenzenes in zeolite micropores, disfavoring the propagation of the aromatic-based catalytic cycle. This conclusion is consistent with a recent report on ZSM-5 and is also strongly supported by our analysis of soluble coke species residing in the catalysts. Moreover, we identified an oxygen-containing compound, 4-methylbenzaldehyde, in the coke, which has not been observed in the MTH reaction before.