

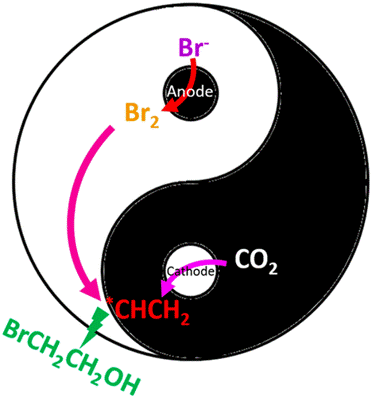
Tremendous research efforts have been made to electrochemically convert CO2 into useful chemicals, with the main products being limited to hydrocarbons and oxygenates. One-step processes that integrate CO2 reduction with a subsequent reaction to produce other types of functional chemicals are economically attractive. Here we report that direct electrochemical conversion of CO2 to 2-bromoethanol, a valuable pharmaceutical intermediate, is enabled by coupling the anodic and cathodic reactions with the presence of potassium bromide electrolyte in a membraneless electrochemical cell. The maximum Faradaic efficiency of converting CO2 to 2-bromoethanol that we achieved is 40% at −1.01 VRHE with a partial current density of −19 mA cm–2. Our work demonstrates a new strategy for making value-added products from CO2 through a simple process.