

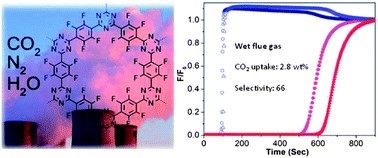
 We designed and synthesized a perfluorinated covalent triazine-based framework (FCTF-1) for selective CO2 capture. The incorporation of fluorine (F) groups played multiple roles in improving the framework's CO2 adsorption and separation capabilities. Thermodynamically, the strongly polar C–F bonds promoted CO2 adsorption via electrostatic interactions, especially at low pressures. FCTF-1's CO2 uptake was 1.76 mmol g−1 at 273 K and 0.1 bar through equilibrium adsorption, exceeding the CO2 adsorption capacity of any reported porous organic polymers to date. In addition, incorporating F groups produced a significant amount of ultra-micropores (<0.5 nm), which offered not only high gas adsorption potential but also kinetic selectivity for CO2–N2 separation. In mixed-gas breakthrough experiments, FCTF-1 exhibited an exceptional CO2–N2 selectivity of 77 under kinetic flow conditions, much higher than the selectivity (31) predicted from single-gas equilibrium adsorption data. Moreover, FCTF-1 proved to be tolerant to water and its CO2 capture performance remained excellent when there was moisture in the gas mixture, due to the hydrophobic nature of the C–F bonds. In addition, the moderate adsorbate–adsorbent interaction allowed it to be fully regenerated by pressure swing adsorption processes. These attributes make FCTF-1 a promising sorbent for CO2 capture from flue gas.
We designed and synthesized a perfluorinated covalent triazine-based framework (FCTF-1) for selective CO2 capture. The incorporation of fluorine (F) groups played multiple roles in improving the framework's CO2 adsorption and separation capabilities. Thermodynamically, the strongly polar C–F bonds promoted CO2 adsorption via electrostatic interactions, especially at low pressures. FCTF-1's CO2 uptake was 1.76 mmol g−1 at 273 K and 0.1 bar through equilibrium adsorption, exceeding the CO2 adsorption capacity of any reported porous organic polymers to date. In addition, incorporating F groups produced a significant amount of ultra-micropores (<0.5 nm), which offered not only high gas adsorption potential but also kinetic selectivity for CO2–N2 separation. In mixed-gas breakthrough experiments, FCTF-1 exhibited an exceptional CO2–N2 selectivity of 77 under kinetic flow conditions, much higher than the selectivity (31) predicted from single-gas equilibrium adsorption data. Moreover, FCTF-1 proved to be tolerant to water and its CO2 capture performance remained excellent when there was moisture in the gas mixture, due to the hydrophobic nature of the C–F bonds. In addition, the moderate adsorbate–adsorbent interaction allowed it to be fully regenerated by pressure swing adsorption processes. These attributes make FCTF-1 a promising sorbent for CO2 capture from flue gas.